Abstract
Introduction: Studies have found that AML pts treated at high pt volume, academic or NCI-designated cancer centers have improved outcomes compared to pts treated at smaller community hospitals. But little is known about the treatment patterns and outcomes as related to a combined academic and community based health system. Therefore, in a real-world cohort that included both academic and community hospitals that collaborate with one another, we evaluated pt characteristics, frequency of genomic testing, frequency of chemotherapy treatment (Tx) or any targeted therapy as a function of age, and outcomes in ND AML pts.
Methods: This was a retrospective observational study of patients treated within a comprehensive health system in the Midwest US that includes both metropolitan and rural populations. Pts ≥18 years (yrs) with ND AML between 1 Jan 2011 and 31 Dec 2018 were identified using ICD-9 / ICD-10 codes for AML within the Health System or from one of two local cancer registries, with a follow-up period that ended with the pt's death, 2 years after initial diagnosis or 31 Dec 2018, whichever came first. Date of diagnosis served as the index date. Kaplan-Meier estimates were used to visualize overall survival.
Results: A total of 629 pts with ND AML were identified in the 3 data sources and included in the cohort for analysis (Figure 1). At the index date, majority (55%) of the pts were older (61 yrs or more), male (55%) and White (89%) (Table 1). Majority of the identified pts (76%) died before the end of the study; higher proportion of older pts died vs younger pts (≥75 yrs: 89%; 61 - 74 yrs: 85% vs. ≤60 yrs: 62%). Most common comorbidities were renal disease (30%), cardiovascular diseases (24%), and diabetes (18%).
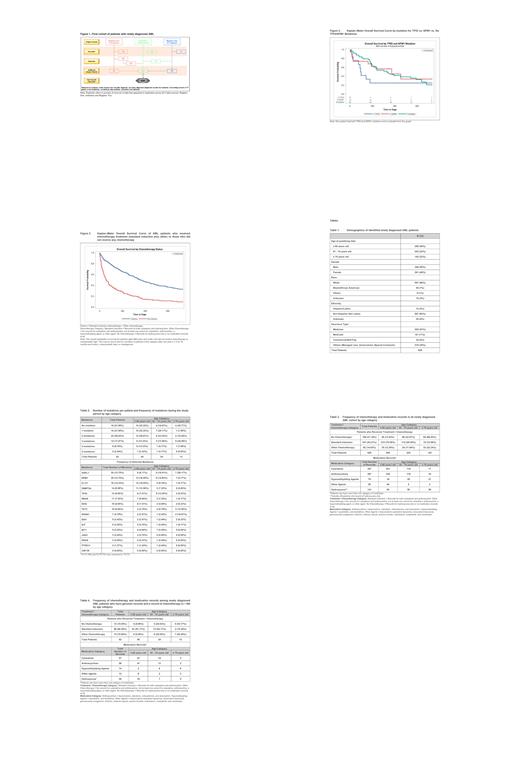
Of the 500 pts with available cytogenetics data, majority had ELN-defined intermediate risk (49%), then adverse (25%) and favorable (6%); no pt ≥75 yrs had favorable risk profile. Only 82 pts (13%) had evidence of a sequencing genomic report; 78% of the pts had at least 1 mutation and 56% had 2 - 5 mutations (Table 2). By age, pts ≥75 yrs had the highest proportion of multiple (≥3) mutations (46%); most common overall were ASXL1, NPM1, and FLT3. By age, mutations with the highest frequencies were: ≥75 yrs - ASXL1, RUNX1 and TET2; 61 - 74 yrs - ASXL1, NPM1 and TP53; and ≤60 yrs - NPM1, FLT3 and DNMT3A. As expected, pts with TP53 mutations had a lower overall probability of survival vs pts with wild type TP53 or NPM1 mutations (Figure 2).
Overall, 69% of pts had records for either standard induction chemotherapy (IC) or other chemotherapy (OC; includes targeted therapy) during the study period; 31% did not have records for chemotherapy (Table 3). Of the 54% of pts with IC, majority (63%) were ≤60 yrs. A higher proportion of pts ≥75 yrs (23%) received OC, which includes hypomethylating agents (HMAs), but 66% of pts ≥75 yrs did not have records for receiving any chemotherapy. Overall probability of survival for pts who received Tx (IC + OC) was higher than for pts who did not receive any Tx (Figure 3). Pts ≥75 years received proportionally more HMAs vs other age groups (Table 3). Anthracyclines and cytarabine records were similar within each age group, suggesting they were given together. In pts with genomic data, 84% received chemotherapy (IC 68%; OC 16%) while 16% did not (Table 4); proportion of pts ≤60 yrs who received IC (91%) was much higher in those with a genomic report than for the entire cohort (75%), and fewer older pts ≥75 yrs with a genomic report did not receive any chemotherapy (31%) vs 66% for the entire cohort.
Conclusions: Results of this retrospective study showed 55% of the ND AML pts were >60 yrs, younger pts (≤60 yrs) are more likely to receive IC and 66% of those ≥75 yrs did not receive any chemotherapy or alternative treatment. Additionally, only 13% of pts had evidence of a genomic report although it has been used for prognostication for at least the last decade. With newer Tx options including targeted therapies, access to genomic analysis and Tx needs to increase across all environments (rural and metropolitan) given the impact that such treatments can have on patient outcome.
Byrd: Vincerx Pharmaceuticals: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, Syndax: Consultancy, Honoraria; Newave: Membership on an entity's Board of Directors or advisory committees. Mims: Leukemia and Lymphoma Society's Beat AML clinical study: Consultancy, Research Funding; Aptevo: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Glycomemetics: Research Funding; Kartos Pharmaceuticals: Research Funding; Xencor: Research Funding; Genentech: Consultancy; Abbvie: Consultancy; BMS: Consultancy; Kura Oncology: Consultancy; Syndax Pharmaceuticals: Consultancy; BMS: Consultancy; Jazz Pharmaceuticals: Consultancy; Aptevo: Research Funding. Borate: AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicine: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rampal: Membership on an entity's Board of Directors or advisory committees; Galecto, Inc.: Consultancy; Promedior: Consultancy.